menu
PRIMS is a complete compliance and safety software solution for cosmetics brands, (3rd party) manufacturers, and consultants.
It helps you manage raw materials, formulas, products, PIFs, Safety Assessments and REACH work in one connected eco-system. This 24/7 accessible cloud platform gives you real-time insights in full portfolio safety and compliance against the latest regulatory and toxicology data.
In this way, your teams can work with a single source of truth for compliance in the EU, UK, USA MoCRA and Canada, including retailer restrictions.
To explore all features in depth and request a demo, visit the PRIMS website.
PRIMS software solution is developed and continuously updated by The Regulatory Company’s Safety Assessors, Toxicologists and Regulatory Specialists.
Specifications automation
PRIMS organises raw materials, formulas, packaging and finished products in one connected system. Each item has its own versioning, lifecycle control and full traceability down to INCI level. Cloning tools speed up product updates and variations. The PIF completeness view highlights missing documents so files are ready for audits and market launch. You can choose to work with API connections to your system.
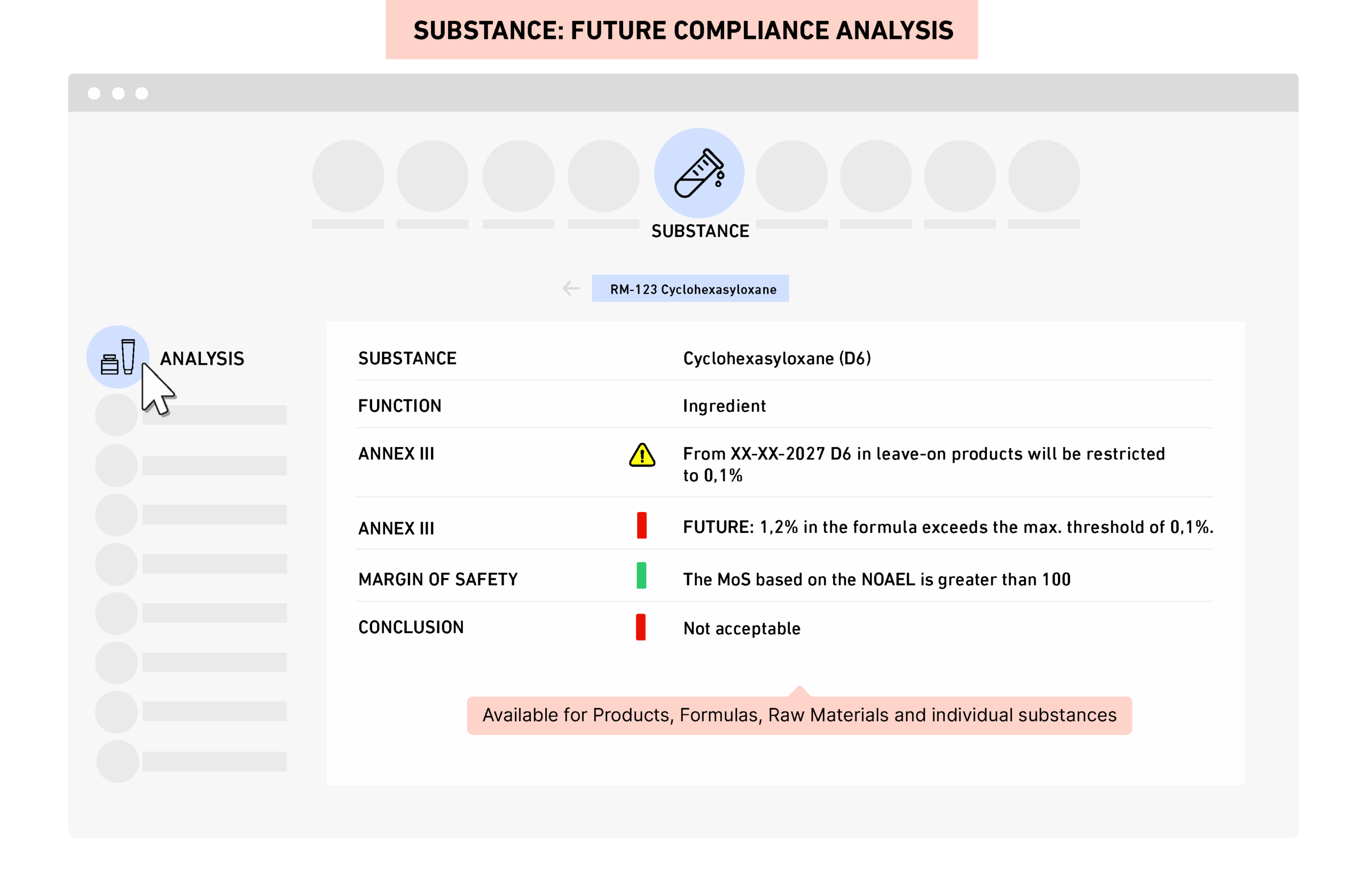
Regulatory and toxicology data, smart algorithmsSubstance level regulatory and toxicology datasets are maintained by The Regulatory Company experts and updated on a continuous basis. Predefined exposure scenarios for more than 150 product types support accurate safety work. Tags such as microplastics, PFAS, Formaldehyde donors and VOCs enable quick portfolio checks. Compliance status reflects current rules, new amendments and retailer restrictions.
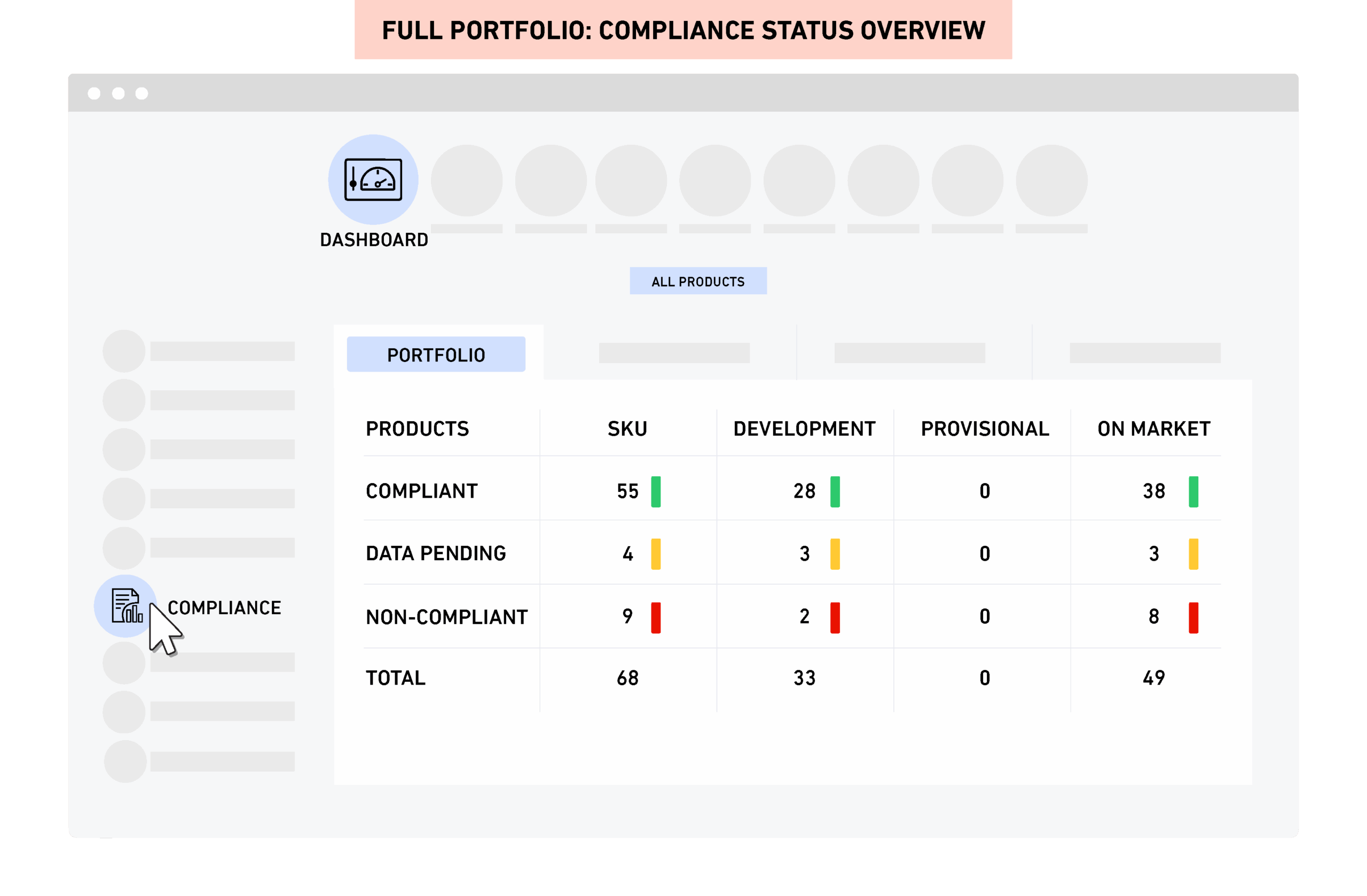
Dashboards and reporting
PRIMS gives real time compliance and safety visibility across the full portfolio. You can access product specific analysis with one click and generate label content and cumulative compositions instantly.You can quickly generate Safety Assessments based on exposure scenarios and toxicology thresholds ready for review and approval.The REACH dashboard calculates imported volumes and thresholds per substance and supplier. "Where-used" views show which products or materials are impacted by regulatory updates.



Contact us and have your portfolio pre-assessed for free! We will look into your needs, your product formulations, your product label and marketing materials, and discuss your product dossiers.
We fully support the data transfer to ensure a smooth transition, a minimum-effort implementation process, and short-term internal adoption of PRIMS.
You provide the compositional data and other structured data in bulk. We perform the data import and we make sure that your PRIMS users are properly trained. You are guided in the entire on-boarding process by one of our dedicated project managers that will be assigned to your project.
Already on the first access to PRIMS most features and reporting are immediately available, such as automated compliance status monitoring and detailed analysis per product, product composition downloads and mandatory label content briefs, 24/7 real-time compliance dashboard (including the REACH management dashboard), and where used reporting.
Faster - bulk import of raw materials and formulations data; intuitive versioning and clone functionality for all specifications; automated compliance and safety analysis; one-click generation of full mandatory label content and all PIF and registrations documents in pdf; instant creation of REACH reports.
Less resources - less work or no data duplication with "one specification in one place"; all specifications are linked with automated data synchronisation enabling “one to all” changes; extensive and easy impact analysis and reporting; dashboards to monitor portfolio compliance and PIF completeness; seamless real-time PIF, CPSR and label content creation and export.
Higher quality - regulatory and toxicology databases are included, kept up-to-date and managed by The Regulatory Company [TRC] with the latest (future) regulatory and toxicology data; auto-updated product compliance and safety statuses and insight at your finger tips, and "back-in time".
Reliable with Confidence - already thousands of products managed by customers and TRC in PRIMS; ensuring full transparency in your product portfolio in a “from substance to consumer unit” fashion; complementary in-house consultancy, compliance and Responsible Person services; proven success in passing competent authority inspections.
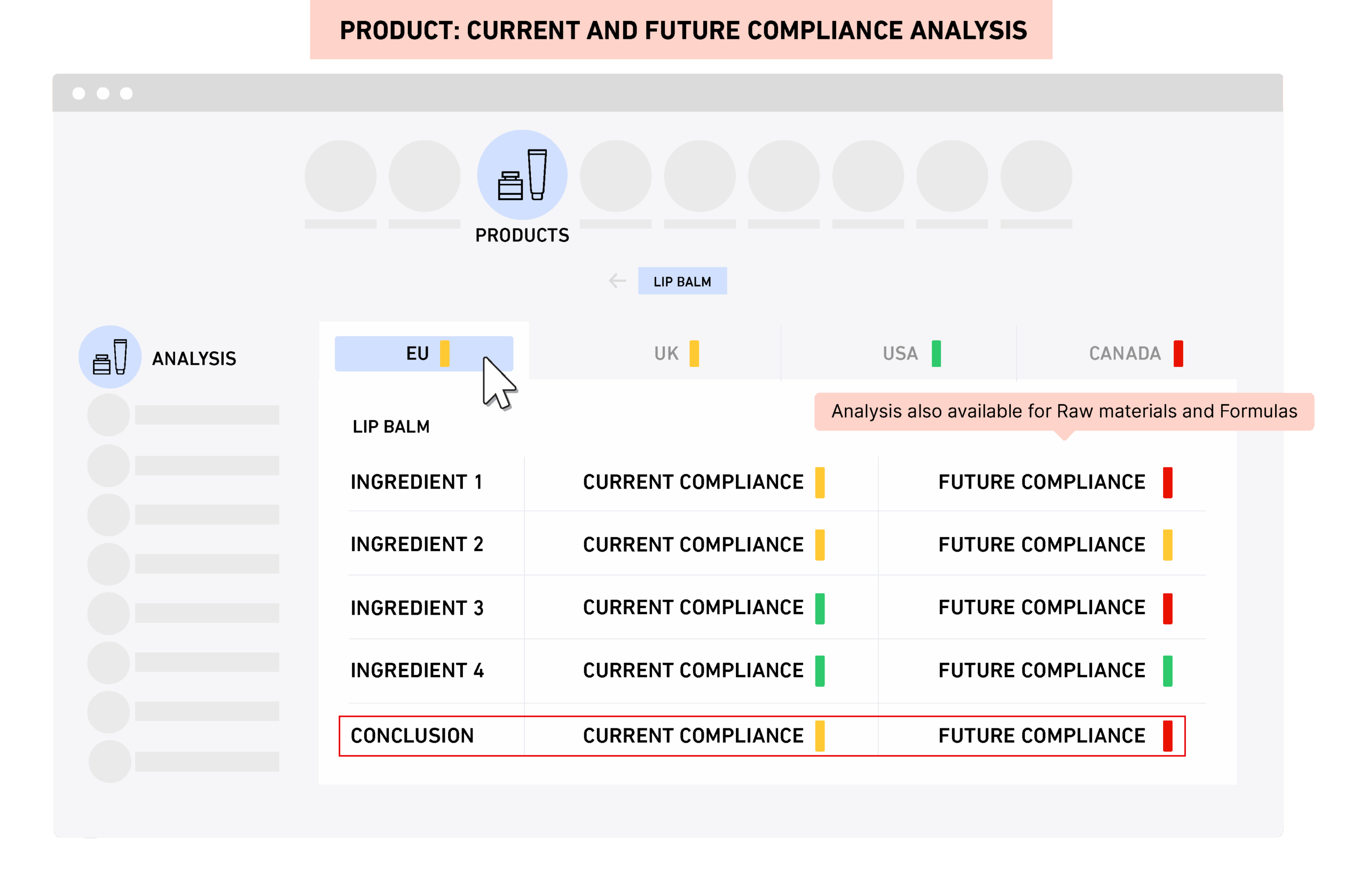
PRIMS includes an intuitive user interface, dashboard overview with product portfolio compliance status and easy navigation. Specifications are fully traceable back and forth through one-click navigation between raw materials, formulations, packaging components, products and companies (suppliers), and where-used reports are generated and exported in spreadsheet format in a few seconds.
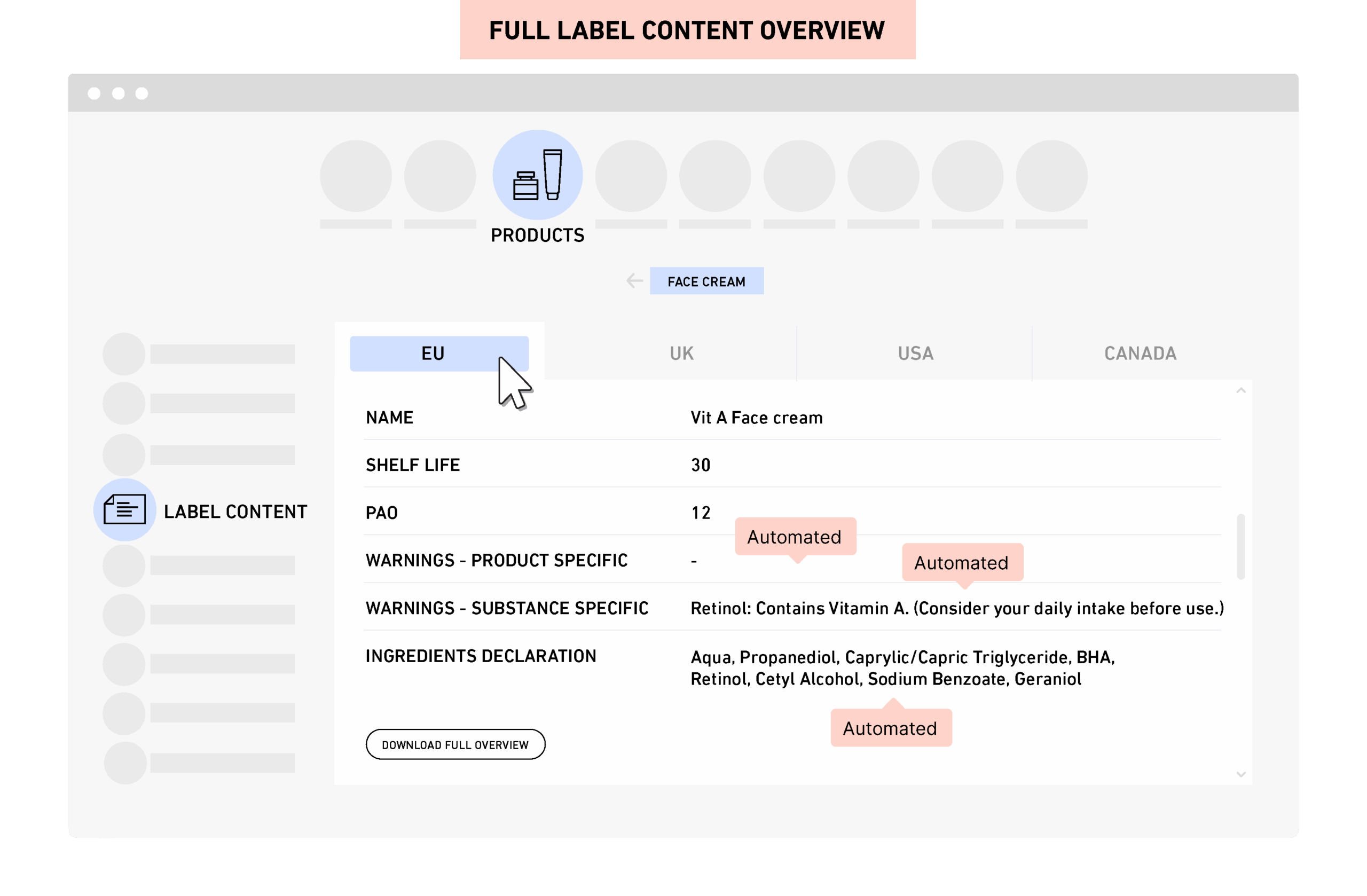
PRIMS has been developed with a 100% focus on regulatory requirements and processes, and to quickly produce the relevant output documents and reports such as the CPSR, Cumulative Product Composition (exact or ranges), and the Regulatory Content Brief (mandatory label content).
Documents and reports are available in PRIMS at all times, are quickly (re-) generated to adjust for any changes, and ready for export/download to all users.
The REACH management dashboard presents a real-time overview of all substance-supplier combinations that require verification of REACH registration compliance.
To manage importer obligations, PRIMS algorithms calculate and present substance-supplier combinations where the annually imported quantity surpasses the 1.000 kg threshold, by at least one individual importer, and where the substance is not exempted.
By changing the selection criteria (calendar year, region, local manufacturers), the dashboard overview is modified to meet your needs.
PRIMS enables the regulatory team to work faster and more efficiently, and adds significant quality, continuity and confidence to the regulatory function. The compliance and safety results are highly reliable.
Once operational (within 4-8 weeks), total resources spent on regulatory processes are significantly reduced with up to 40%, in particular on PIF management, and (continuous) compliance and safety assessment.
